Brain-derived neurotrophic factor, mature (BDNF, mature), Human, CE-Marked ELISA assay
- Product Name Brain-derived neurotrophic factor, mature (BDNF, mature), Human, CE-Marked ELISA assay
-
Product Description
The Biosensis CE Marked BDNF Rapid enzyme-linked immunosorbent assay (ELISA) Kit is a sandwich ELISA that allows the preferential quantification of mature BDNF in less than 3 hours. This kit consists of a pre-coated mouse monoclonal anti-BDNF capture antibody, a biotinylated anti-BDNF detection antibody and horseradish peroxidase (HRP)-conjugated streptavidin. The addition of a substrate (3,3',5,5'-tetramethylbenzidine, TMB) yields a colored reaction product which is directly proportional to the concentration of BDNF present in samples and protein standards. This BDNF ELISA kit employs a recombinant human BDNF standard approved by the World Health Organization (WHO, www.nibsc.org). This kit is suitable to measure mature BDNF in human serum and citrate-treated plasma samples only. The antibodies used in this ELISA kit bind epitopes within the mature domain of the protein and therefore recognize the mature as well as the pro-form of BDNF. However, cross-reactivity to the full-length proBDNF protein is low.
This CE Marked BDNF Rapid ELISA [Cat. No. BEK-2211-CE] Kit is approved for in-vitro diagnostic (IVD) applications in the European Economic Area (EEA). It has been developed by Biosensis and is manufactured by Calbiotech Inc. (www.calbiotech.com) for Biosensis.
BEK-2211-CE is not approved for in-vitro diagnostic (IVD) applications in the United States. For research on human blood, customers MUST order the catalog number BEK-2211. This research-use-only ELISA kit can be used for human and animal research purposes worldwide, and has been validated for a wider range of sample types and species. - Alternative Names Brain-derived neurotrophic factor; BDNF; Abrineurin;
- Application(s) ELISA
- Specificity Human BDNF when used as directed.
- Species Reactivity Human
- Immunogen Description Recombinant human BDNF with an N-terminal methionine residue, made in E. coli (WHO reference reagent)
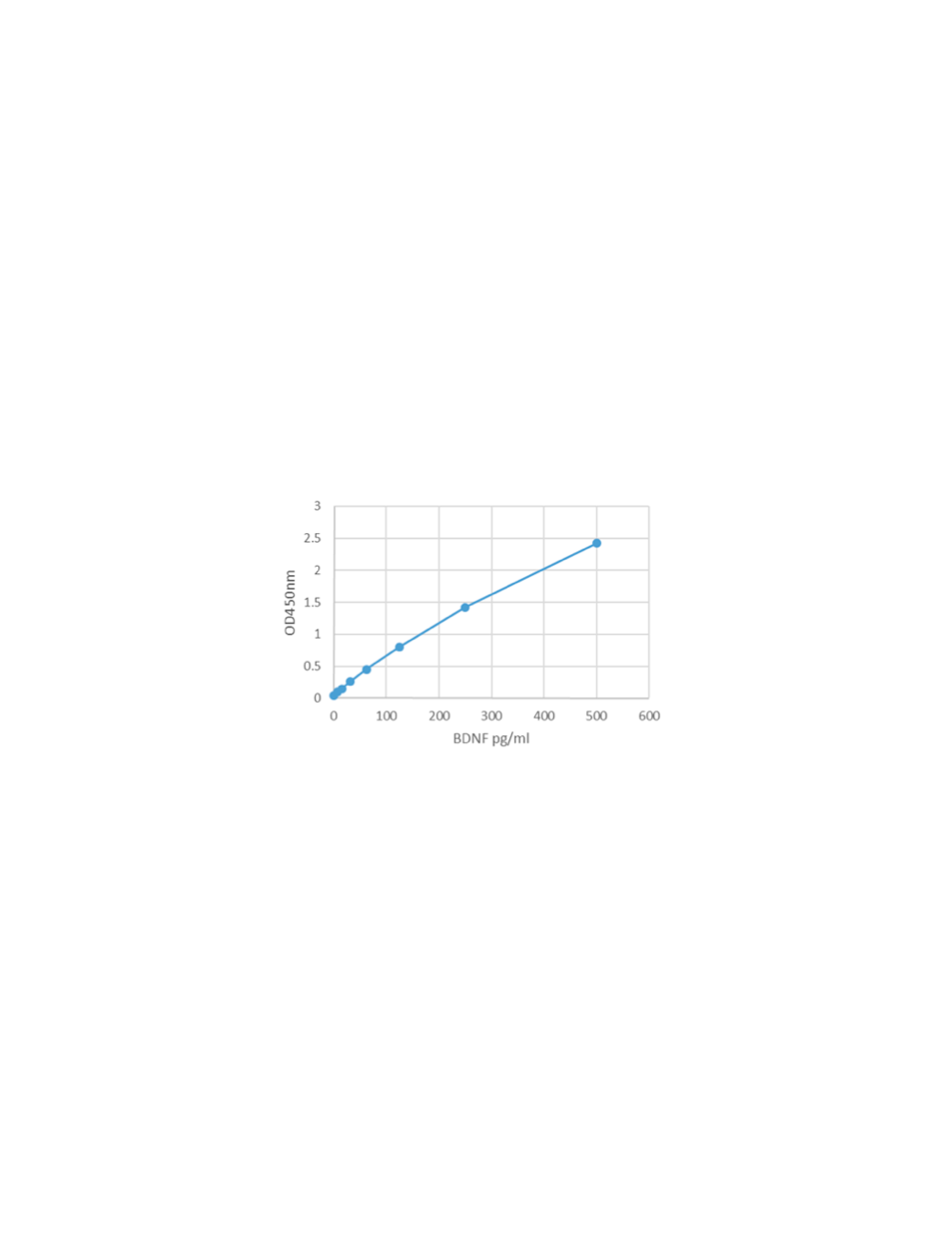
- Range 7.8 pg/mL - 500 pg/mL
- Sensitivity Typical limit of detection (LOD) for BDNF is < 3 pg/mL determined by calculating the mean + 2x standard deviation of mean of blank (n=20).
- Regulatory Status Approved for in-vitro diagnostic (IVD) applications in the European Economic Area (EEA). It has been developed by Biosensis and is manufactured by Calbiotech Inc. (www.calbiotech.com) for Biosensis. This kit is not approved for in-vitro diagnostic (IVD) applications in the United States. For research on human blood, customers MUST order the catalog number BEK-2211.
Product Info
-
Product Description
The Biosensis CE Marked BDNF Rapid enzyme-linked immunosorbent assay (ELISA) Kit is a sandwich ELISA that allows the preferential quantification of mature BDNF in less than 3 hours. This kit consists of a pre-coated mouse monoclonal anti-BDNF capture antibody, a biotinylated anti-BDNF detection antibody and horseradish peroxidase (HRP)-conjugated streptavidin. The addition of a substrate (3,3',5,5'-tetramethylbenzidine, TMB) yields a colored reaction product which is directly proportional to the concentration of BDNF present in samples and protein standards. This BDNF ELISA kit employs a recombinant human BDNF standard approved by the World Health Organization (WHO, www.nibsc.org). This kit is suitable to measure mature BDNF in human serum and citrate-treated plasma samples only. The antibodies used in this ELISA kit bind epitopes within the mature domain of the protein and therefore recognize the mature as well as the pro-form of BDNF. However, cross-reactivity to the full-length proBDNF protein is low.
This CE Marked BDNF Rapid ELISA [Cat. No. BEK-2211-CE] Kit is approved for in-vitro diagnostic (IVD) applications in the European Economic Area (EEA). It has been developed by Biosensis and is manufactured by Calbiotech Inc. (www.calbiotech.com) for Biosensis.
BEK-2211-CE is not approved for in-vitro diagnostic (IVD) applications in the United States. For research on human blood, customers MUST order the catalog number BEK-2211. This research-use-only ELISA kit can be used for human and animal research purposes worldwide, and has been validated for a wider range of sample types and species. - Application(s) ELISA
- Application Details ELISA. For the quantification of Brain-derived neurotrophic factor, mature (BDNF, mature) in Serum, Plasma (Citrate). BEK-2211-CE is expressly designed and tested only for use on human blood and plasma samples. Any other use is deemed "off label use" and thus the performance characteristics of the assay will have to be determined by the end user, and such results are not supported by Biosensis or CalBioTech at this time. Please download the detailed product insert for complete instructions for the successful use of this ELISA. Use only as directed.
- Target Brain-derived neurotrophic factor, mature (BDNF, mature)
- Specificity Human BDNF when used as directed.
- Target Host Species Human
- Species Reactivity Human
- Immunogen Description Recombinant human BDNF with an N-terminal methionine residue, made in E. coli (WHO reference reagent)
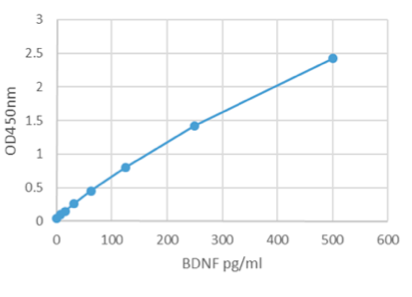
- Range 7.8 pg/mL - 500 pg/mL
- Sensitivity Typical limit of detection (LOD) for BDNF is < 3 pg/mL determined by calculating the mean + 2x standard deviation of mean of blank (n=20).
- Sample Type Plasma (Citrate), Serum
- Detection Method Colorimetric
- Kit Components The ELISA kit box contains 96-well pre-coated strip plate(s), protein standards, detection reagents, wash and sample buffers, substrate buffer and detailed protocols.
- Storage Instructions Store at 2-8°C
- Storage Temperature 2-8°C
- Batch Number Please see item label.
- Expiration Date See BEK-2211-2P-CE protocol insert for specific expiration dating of the kit and its components.
- Alternative Names Brain-derived neurotrophic factor; BDNF; Abrineurin;
- Uniprot Number P23560
- Uniprot Number/Name P23560 (BDNF_HUMAN)
- Scientific Background BDNF belongs to the neurotrophin family and regulates the survival and differentiation of neurons during development. The alterations in BDNF expression induced by various kinds of brain insult including stress, ischemia, seizure activity and hypoglycemia, may contribute to some pathologies such as depression, epilepsy, Alzheimer's, and Parkinson's disease. FUNCTION: Promotes the survival of neuronal populations that are all located either in the central nervous system or directly connected to it. Major regulator of synaptic transmission and plasticity at adult synapses in many regions of the CNS. The versatility of BDNF is emphasized by its contribution to a range of adaptive neuronal responses including long-term potentiation (LTP), long-term depression (LTD), certain forms of short-term synaptic plasticity, as well as homeostatic regulation of intrinsic neuronal excitability. SUBUNIT: Monomers and homodimers. Binds to NTRK2/TRKB. SUBCELLULAR LOCATION: Secreted protein. Post Translation Modification (PTM): The propeptide is N-glycosylated and glycosulfated. PTM: Converted into mature BDNF by plasmin (PLG) (By similarity). DISEASE: Defects in BDNF are a cause of congenital central hypoventilation syndrome (CCHS); also known as congenital failure of autonomic control or Ondine curse. CCHS is a rare disorder characterized by abnormal control of respiration in the absence of neuromuscular or lung disease, or an identifiable brain stem lesion. A deficiency in autonomic control of respiration results in inadequate or negligible ventilatory and arousal responses to hypercapnia and hypoxemia. CCHS is frequently complicated with neurocristopathies such as Hirschsprung disease that occurs in about 16% of CCHS cases. SIMILARITY: Belongs to the NGF-beta family.
- Shipping Statement For order quantities larger than 1 plate, this product is supplied in package units of 2 plates.
- Shipping Temperature 2-8°C (on cold packs)
- UNSPSC CODE 41116158
- Regulatory Status Approved for in-vitro diagnostic (IVD) applications in the European Economic Area (EEA). It has been developed by Biosensis and is manufactured by Calbiotech Inc. (www.calbiotech.com) for Biosensis. This kit is not approved for in-vitro diagnostic (IVD) applications in the United States. For research on human blood, customers MUST order the catalog number BEK-2211.
Specifications
-
Specific References
Kim N et al. (2024) Formulated Palmitoylethanolamide Supplementation Improves Parameters of Cognitive Function and BDNF Levels in Young, Healthy Adults: A Randomised Cross-Over Trial; Nutrients, 16(4), 489. Application: Human serum
Reed JL et al. (2021) The effects of high-intensity interval training, Nordic walking and moderate-to-vigorous intensity continuous training on functional capacity, depression and quality of life in patients with coronary artery disease enrolled in cardiac rehabilitation: A randomized controlled trial (CRX study). Prog Cardiovasc Dis. [Epub ahead of print] Application: Human blood.
Valkenborghs SR et al. (2019) Aerobic exercise and consecutive task-specific training (AExaCTT) for upper limb recovery after stroke: A randomized controlled pilot study Physiother Res Int. [Epub ahead of print]. Application: Human serum.br>

 1800 605-5127
1800 605-5127 +61 (0)8 8352 7711
+61 (0)8 8352 7711