Aldolase C, Mouse Monoclonal Antibody
- Product Name Aldolase C, Mouse Monoclonal Antibody
-
Product Description
Mouse anti-Aldolase C Monoclonal Antibody (Unconjugated), suitable for WB, ICC.
- Alternative Names Brain-type aldolase, Fructose-bisphosphate aldolase C
- Application(s) ICC, WB
- Antibody Host Mouse
- Antibody Type Monoclonal
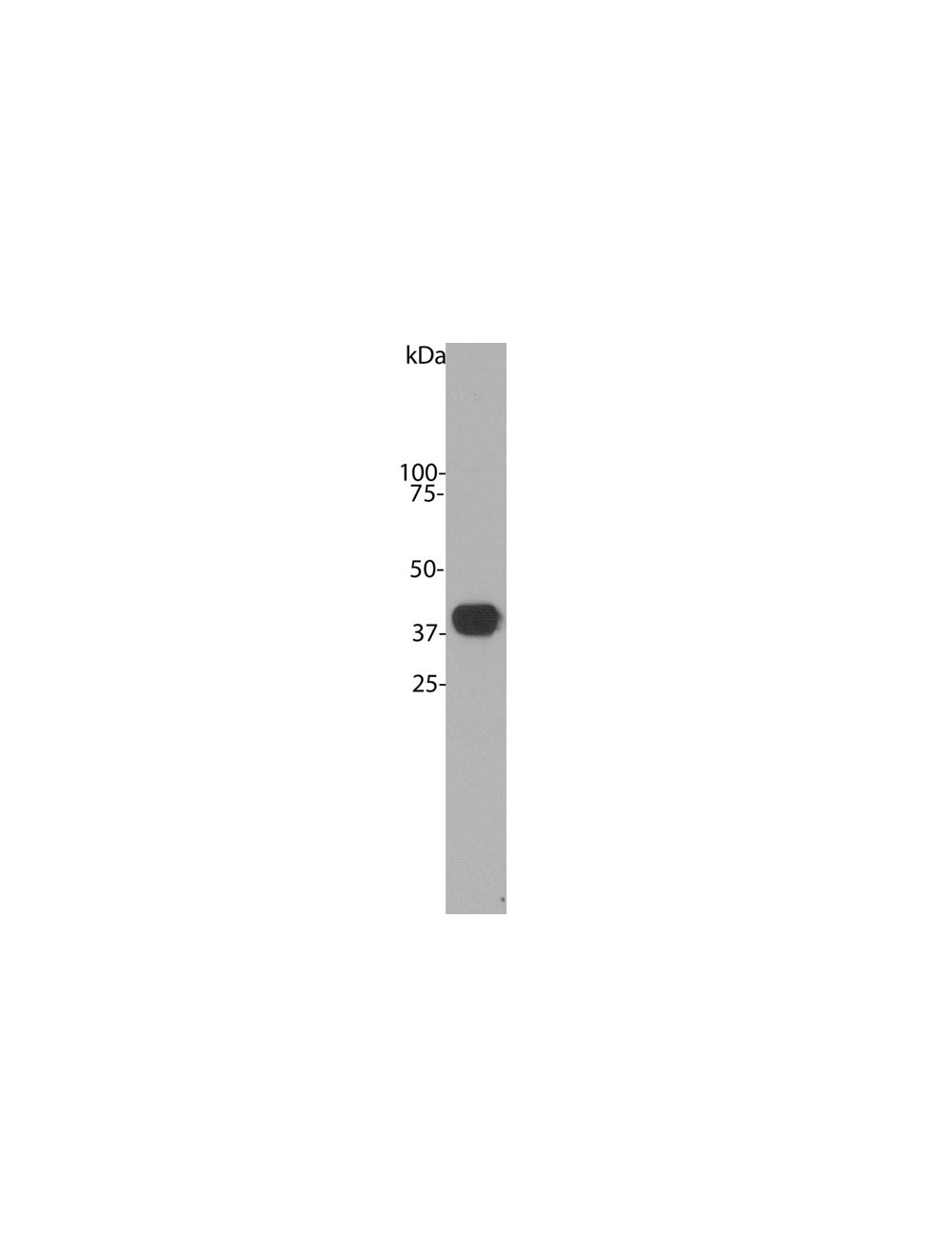
- Specificity The antibody reacts with a 40 kDa band by Western blot on a crude bovine cerebellum homogenate. It has also been used successfully for immunocytochemistry.
- Species Reactivity Bovine, Human, Mouse, Pig, Rat
- Immunogen Description N-terminal 20 amino acids of aldolase C protein, MPHSYPALSAEQKKELSDIA
- Conjugate Unconjugated
- Purity Description Protein G purified
- Regulatory Status For research use only.
Product Info
-
Product Description
Mouse anti-Aldolase C Monoclonal Antibody (Unconjugated), suitable for WB, ICC.
- Application(s) ICC, WB
- Application Details Western Blotting (WB) and Immunocytochemistry (ICC). A dilution of 1:1,000 - 1:2,000 is recommended for WB. A dilution of 1:500 - 1:1,000 is recommended for IC. Biosensis recommends optimal dilutions/concentrations should be determined by the end user.
- Target Aldolase C
- Specificity The antibody reacts with a 40 kDa band by Western blot on a crude bovine cerebellum homogenate. It has also been used successfully for immunocytochemistry.
- Target Host Species Human
- Species Reactivity Bovine, Human, Mouse, Pig, Rat
- Antibody Host Mouse
- Antibody Type Monoclonal
- Antibody Isotype IgG1
- Clone Name 4A9
- Conjugate Unconjugated
- Immunogen Description N-terminal 20 amino acids of aldolase C protein, MPHSYPALSAEQKKELSDIA
- Purity Description Protein G purified
- Format Lyophilized from PBS buffer pH 7.2-7.6 with 0.1% trehalose, and sodium azide
- Reconstitution Instructions Spin vial briefly before opening. Reconstitute with 100 µL sterile-filtered, ultrapure water to achieve a 1 mg/mL concentration. Centrifuge to remove any insoluble material.
- Storage Instructions After reconstitution of lyophilized antibody, aliquot and store at -20°C for a higher stability. Avoid freeze-thaw cycles.
- Batch Number Please see item label.
- Expiration Date 12 months after date of receipt (unopened vial).
- Alternative Names Brain-type aldolase, Fructose-bisphosphate aldolase C
- Uniprot Number P09972
- Uniprot Number/Name P09972 (ALDOC_HUMAN)
-
Scientific Background
Aldolases are glycolytic enzymes that catalyze the reversible aldol cleavage of fructose 1,6-bisphosphate and fructose-1-phosphate to dihydroxyacetone phosphate and either glyceraldehyde 3-phosphate or glyceraldehyde, respectively. Thus, Aldolases play important roles in glycolysis and the reverse pathway, gluconeogenesis, catalyzing the reversible conversion of fructose-1,6-bisphosphate to glyceraldehydes-3-phosphate (G3P) or glyceraldehyde and dihydroxyacetone phosphate (DHAP). At this step, Adolases are key regulators of ATP biosynthesis. Three aldolase isozymes are found in mammals, specifically aldolases A, B, and C, each of which is encoded by a separate gene. Aldolase A is generally considered to be a muscle enzyme. Northern analysis of cultured cells suggests that it is also present in both neurons and glia (1). Aldolase B is considered to be a liver-specific enzyme, and it is transcriptionally activated by signals from hormones and dietary factors (2). In adults, aldolase C is generally considered a brain-specific isozyme, with low but detectable activity in fetal tissues (1, 3-6). Aldolase C shares 81% amino acid identity with aldolase A and 70% identity with aldolase B. Earlier studies using isozyme-specific antibodies report its location in gray matter astrocytes and cells of the pia mater (5, 8). In situ hybridization of mouse central nervous system using isozyme-specific probes revealed that aldolase A and C are expressed in complementary cell types: aldolase A mRNA is found in neurons; aldolase C message is detected in astrocytes, some cells of the pia mater, and Purkinje cells (9). Aldolase C can, in some situations, be used as an astrocyte marker. However, Purkinje cells of the cerebellum contain high levels of the enzyme, so the enzyme is not totally astrocyte-specific. Indeed, with today’s advanced expression technologies, Aldolase C has been found to be expressed in most tissues (Reference link.) , with predominant expression in the brain, muscle, and most organ systems except reproductive. Its presence in other cell types, such as platelets and mast cells, maybe a backup if other predominant aldolase isozymes become inactivated. ALDOC is localized within the cytoplasm of cells. In the brain, research has also shown that Aldolase C is activated when the brain develops, is injured, or traumatized.
Aldolase C also potentially contributes to other functions, although these are not fully understood. Notably, Aldolase C binds less tightly to the cytoskeleton, such as F-actin, than other isozymes, possibly due to its more acidic pI. It also plays a role in the stress-response pathway for lung epithelial cell function during hypoxia and in the resistance of cerebellar Purkinje cells against excitotoxic insult.
Clinically, Aldolase C overexpression has been associated with cancer. It is found to be upregulated in the brains of schizophrenia patients, with differential expression in the anterior cingulate cortex of male schizophrenia patients, suggesting different regulatory mechanisms in male versus female patients. More recently, Aldolase C has been found to be important in cholesterol biosynthesis by regulating the intermediate metabolite acetyl-CoA. Aldoc may be a novel therapeutic target for reducing the conversion of refined carbohydrates to cholesterol and, therefore, reduce CVD risk (Reference link.)
In the context of neurodegenerative diseases, Adolase C is reported to undergo oxidation in brains affected by mild cognitive impairment (MCI) and Alzheimer's disease (AD). This oxidative modification inhibits Aldolase C activity, leading to the accumulation of fructose 1,6- bisphosphate and driving the reverse reaction towards gluconeogenesis rather than glycolysis, thereby halting ATP production.
In summary, the role of Aldolases, particularly Aldolase C, extends beyond glycolytic processes to underpin key functions across various cellular contexts. Aldolase C, while prominently expressed in the brain, muscle, and other organ systems, serves an intricate role in cellular energy regulation, stress responses, and specialized functions in distinct cell types. The dynamic nature of Aldolase C expression, regulation, and activity in many different cell types and emerging diseases calls for further research that will continue to build our understanding and knowledge of this enzyme and that of its vital family members.
- Shipping Temperature 25°C (ambient)
- UNSPSC CODE 41116161
- Regulatory Status For research use only.
Specifications
-
General References
Popovici T, Berwald-Netter Y, Vibert M, Kahn A, Skala H. Localization of aldolase C mRNA in brain cells. FEBS Lett. 268, 189-193 (1990).
Weber A, Marie J, Cottereau D, Simon M, Besmond C, Dreyfus J. & Kahn A. Dietary Control of Aldolase B and L-type Pyruvate Kinamse RNAs in Rat. J. Biol. Chem 259, 1798-1802 (1984).
Mukai T, Yatsuki H, Masuko S, Arai Y, Joh K & Hori K. The structure of the brain-specific rat aldolase C gene and its regional expression. Biochem. Biophys. Res. Commun. 174, 1035-1042 (1991).
Royds J, Ironside J, Warnaar S, Taylor C & Timperle W. Monoclonal antibody to aldolase C: a selective marker for Purkinje cells in the human cerebellum. Neuropathol. Appl. Neurobiol. 13, 11-21(1987).
Thompson R., Kynoch P. Willson V. Cellular localization of aldolase C subunits in human brain. Brain Res. 232, 489-493 (1982).
Schapira F, Reuber M, Hatzfeld A. Resurgence of two fetal-type of aldolases (A and C) in some fast-growing hepatomas. Biochem. Biophys. Res. Commun. 40, 321-327(1970).
Arai Y, Kajihara S, Masuda J, Ohishi S, Zen K, Ogata J. Mukai T. Position-independent, high-level, and correct regional expression of the rat aldolase C gene in the central nervous system of transgenic mice. Eur. J. Biochem. 221, 253-260 (1994).
Wachsmuth E, Thorner M. & Pfleiderer G. The cellular distribution of aldolase isozymes in rat kidney and brain determined in tissue sections by the immuno-histochemical method. Histochemistry, 45, 143-161 (1975).
Walther EU, Dichgans M, Maricich SM, Romito RR, Yang F, Dziennis S, Zackson S, Hawkes R, Herrup K. Genomic sequences of aldolase C (Zebrin II) direct lacZ expression exclusively in non-neuronal cells of transgenic mice. Proc Natl Acad Sci U S A. Mar 3;95(5):2615-20(1998).

 1800 605-5127
1800 605-5127 +61 (0)8 8352 7711
+61 (0)8 8352 7711