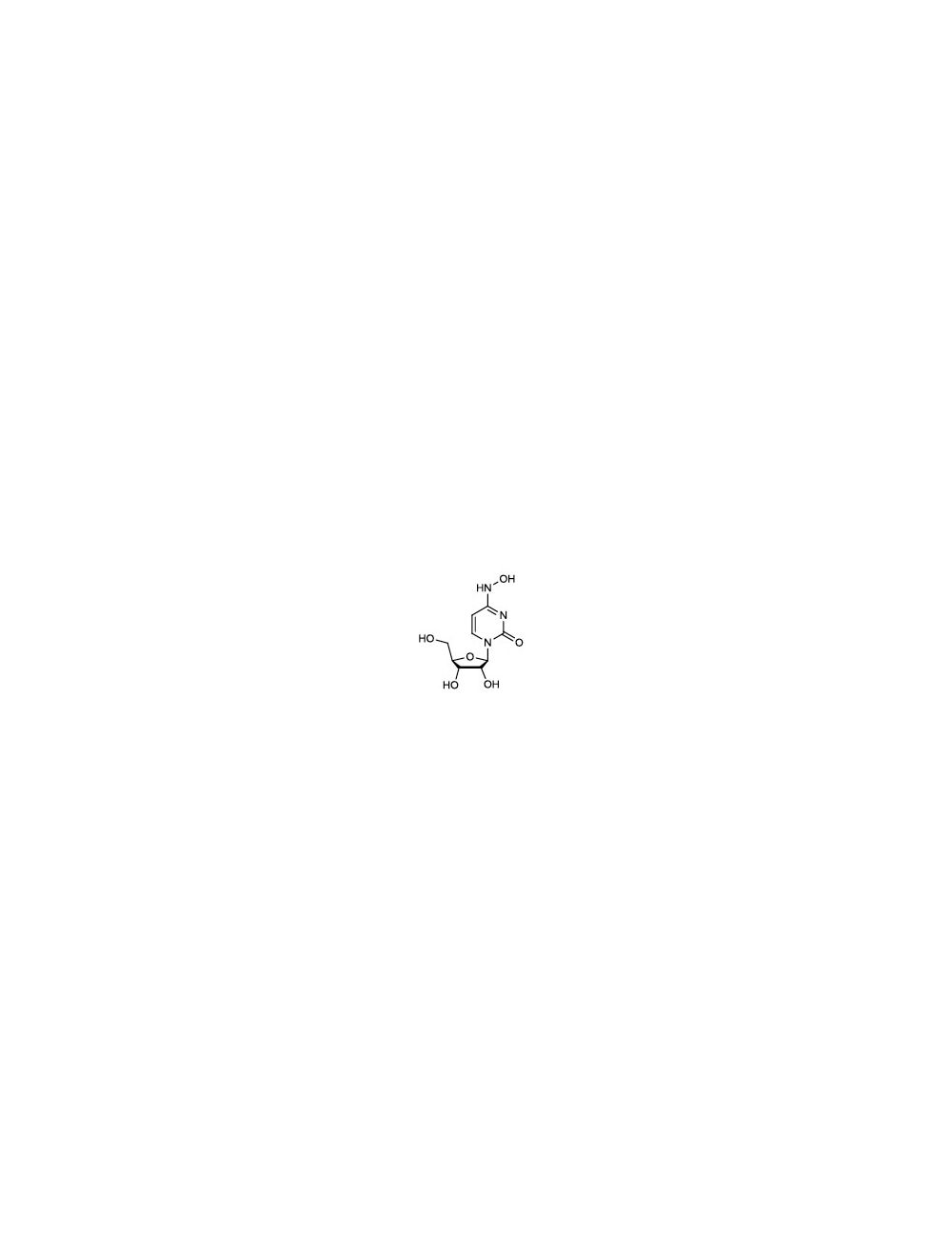
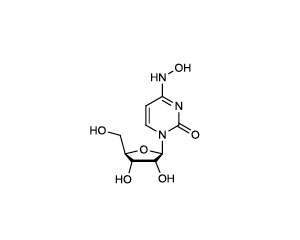
N4-Hydroxycytidine (HC), Purified Small Molecule
Only %1 left
Catalog Number
PE-1845
Discontinued
- Product Name N4-Hydroxycytidine (HC), Purified Small Molecule
- Product Description N4-Hydroxycytidine (HC), Purified Small Molecule, suitable to Neutralize.
- Alternative Names _-D-N4-hydroxycytidine; NHC; CAS 3258-02-4
- Application(s) Neutralize
- Purity Description >98% (HPLC); NMR (Conforms)
- Purity % > 98%
- Regulatory Status For research use only.
Product Info
- Product Description N4-Hydroxycytidine (HC), Purified Small Molecule, suitable to Neutralize.
- Application(s) Neutralize
- Application Details Beta-d-N4-hydroxycytidine (NHC) is a nucleoside analogue that has antipestivirus and antihepacivirus activities; inhibits the production of cytopathic BVDV RNA with EC90 of 5.4 uM, also has an EC50 of 5 uM for replicon RNA reduction in Huh7 cells; also is a novel inhibitor of CHIKV (Chikungunya virus), inhibits venezuelan equine encephalitis virus (VEEV) with EC50 of <1 uM (8-11).
- Target N4-Hydroxycytidine (HC)
- Target Host Species Species Independent
- Purity Description >98% (HPLC); NMR (Conforms)
- Purity % > 98%
- Format Dry, white powder.
- Reconstitution Instructions Soluble in DMSO or slightly in water. May be dissolved in DMSO up to 25 mg/mL; water (15 mg/mL).
- Storage Instructions Store desiccated as supplied at -20°C for up to 2 years. Store solutions at -20°C for up to 1 month. Prevent multiple freeze-thaw cycles.
- Batch Number Please see item label.
- Expiration Date Store desiccated as supplied at -20°C for up to 2 years. Store solutions at -20°C for up to 1 month. Prevent multiple freeze-thaw cycles.
- Alternative Names _-D-N4-hydroxycytidine; NHC; CAS 3258-02-4
-
Scientific Background
Pharmacology: N4-Hydroxycytidine was originally identified as a mutagen effecting AT to GC base-pair transitions (1). It has also been found to have antiviral properties against a broad range of viruses including hepatitis C (2), norovirus (3), Ebola virus (4), influenza and respiratory syncytial viruses (5) and coronaviruses (6). Active molecule in the antiviral pro-drug clinical candidate EIDD-28017. PubChem CID: 197020.
Product is sold for research use only. Not for human therapeutic use or for medicinal purposes.
InChI
InChI=1S/C9H13N3O6/c13-3-4-6(14)7(15)8(18-4)12-2-1-5(11-17)10-9(12)16/h1-2,4,6-8,13-15,17H,3H2,(H,10,11,16)/t4-,6-,7-,8-/m1/s1
InChI Key
XCUAIINAJCDIPM-XVFCMESISA-N
Canonical SMILES
C1=CN(C(=O)N=C1NO)C2C(C(C(O2)CO)O)O
Isomeric SMILES
C1=CN(C(=O)N=C1NO)[C@H]2[C@@H]([C@@H]([C@H](O2)CO)O)O - Shipping Temperature 25°C (ambient)
- UNSPSC CODE 12352401
- Regulatory Status For research use only.
Specifications
-
General References
C Janion and BW Glickman Mutat. Res. 1980 72:43
LJ Stuyver et al. Antimicrob. Agents Chemother. 2003 47:244
VP Costantini et al. Antivir. Ther. 2012 1:981
O Reynard et al. Viruses 2015 7:6233
J-J Yoon et al. Antimicrob. Agents Chemother. 2018 62:e00766-18
K Pyrc et al. Antimicrob. Agents Chemother. 2006 50:2000
M Toots et al. Sci. Transl. Med. 2019 11:eaax5866
Stuyver LJ, et al. Antimicrob Agents Chemother. 2003 Jan;47(1):244-54.
Barnard DL, et al. Antivir Chem Chemother. 2004 Jan;15(1):15-22.
Urakova N, et al. J Virol. 2017 Nov 22. pii: JVI.01965-17.
Ehteshami M, et al. Antimicrob Agents Chemother. 2017 Mar 24;61(4). pii: e02395-16.

 1800 605-5127
1800 605-5127 +61 (0)8 8352 7711
+61 (0)8 8352 7711