SARS-CoV-2 Nucleocapsid protein (NP), Total Human IgG, ELISA assay
Only %1 left
Catalog Number
BEK-1003
Discontinued
- Product Name SARS-CoV-2 Nucleocapsid protein (NP), Total Human IgG, ELISA assay
-
Product Description
The SARS-CoV-2 NP Total Antibody ELISA kit is a highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) for the detection and qualitative measurement of total antibodies against the nucleocapsid protein (NP) of SARS-CoV-2 virus (causing Covid-19) in human serum, plasma (Citrate), plasma (EDTA) and plasma (Heparin).
The Biosensis SARS-CoV-2 NP Total Antibody ELISA kit is a two-step incubation immunoassay kit. Recombinant nucleocapsid protein (NP) of SARS-CoV-2 pre-coated onto the polystyrene microwell strips can specifically recognize anti-NP antibodies in human serum or plasma specimens. After a 1 hour incubation, anti-NP antibodies are captured by immobilized NP protein while the unbound components are washed away. Afterwards, a detection solution containing HRP-conjugated anti-human antibody is added for another 1 hour incubation, wherein HRP-conjugated anti-human antibody binds to the antibodies previously bound to NP protein on the plate. After removal of nonspecific bindings, an HRP substrate solution containing 3,3',5,5'-Tetramethylbenzidine (TMB) is added, resulting in the formation of a blue color. Color reaction is stopped by 2M H2SO4, transforming the blue color to yellow, which is quantified by an absorbance microplate reader at 450 nm. Microplate wells with increased color intensity indicate presence of higher amount of anti-NP antibodies in the tested samples.
This product is intended for use by professional persons only. For research use only. Not for use in clinical & diagnostic procedures. In line with FDA guidelines, test reports must include the following statements:
(1) This test has not been reviewed by the FDA.
(2) Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus.
(3) Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
(4) Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
(5) Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. - Alternative Names Nucleocapsid phosphoprotein [Severe acute respiratory syndrome coronavirus 2]; SARS-CoV-2;
- Application(s) ELISA
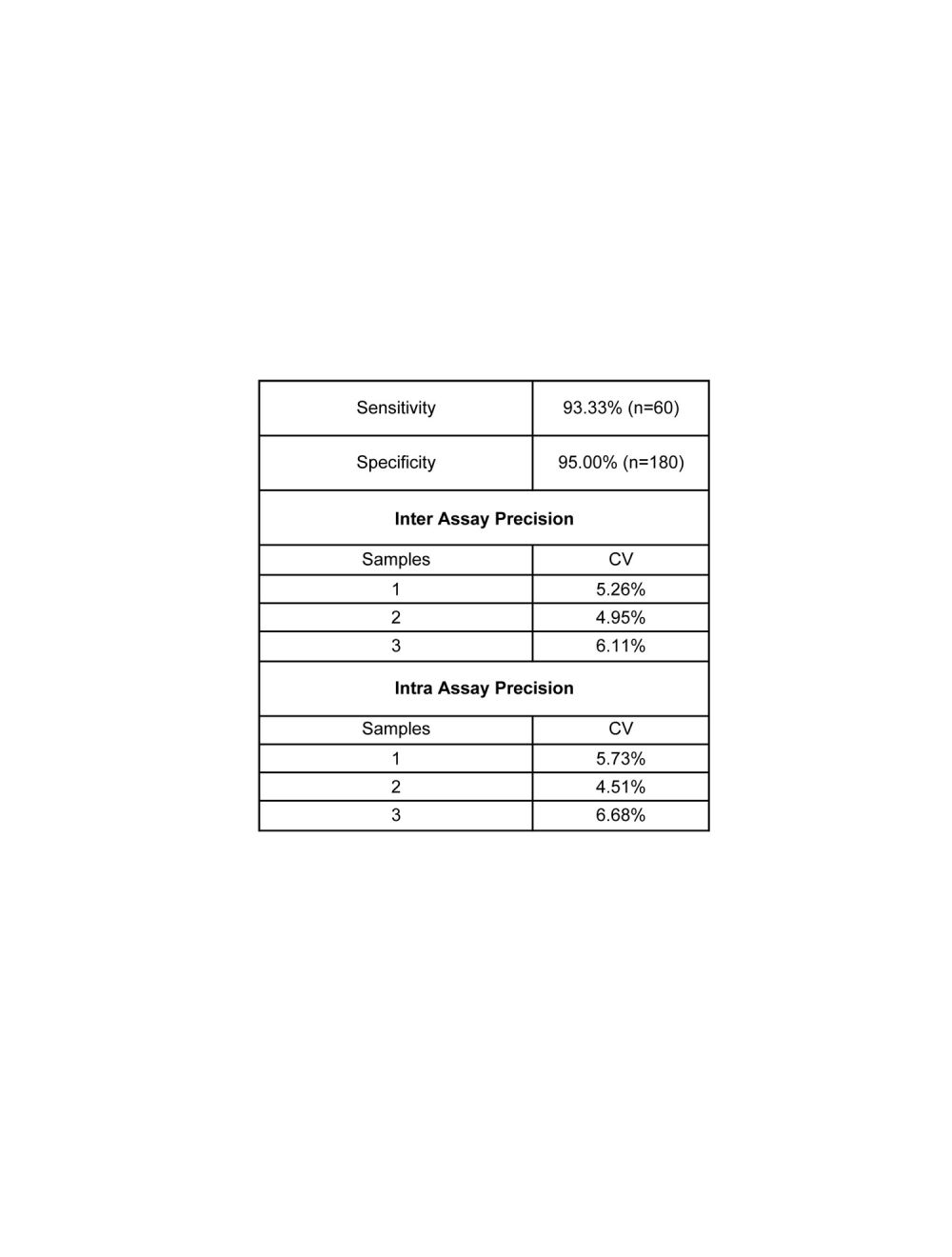
- Specificity Specificity: 95.0 % (n = 180)/Sensitivity: 93.3 % (n = 60);Clinical validation study of the Biosensis SARS-CoV-2 NP Total Antibody ELISA was conducted in 2020 in Shenzhen, China. Samples were collected from COVID-19 confirmed cases with clinical symptoms, laboratory abnormalities or pulmonary imaging manifestations. No tests have been performed on specimens from latent infections or patients in the incubation period. The kit showed higher positive detection rate in specimens from patients with delayed onset. Therefore, the interpretation of the test results should consider the specimen s collection time. It is highly recommended that each laboratory establishes its own normal and pathological reference range for anti-NP antibody level. Furthermore, it is also recommended that each laboratory includes its own panel of control samples in the assay.
- Species Reactivity Human
- Regulatory Status For research use only.
Product Info
-
Product Description
The SARS-CoV-2 NP Total Antibody ELISA kit is a highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) for the detection and qualitative measurement of total antibodies against the nucleocapsid protein (NP) of SARS-CoV-2 virus (causing Covid-19) in human serum, plasma (Citrate), plasma (EDTA) and plasma (Heparin).
The Biosensis SARS-CoV-2 NP Total Antibody ELISA kit is a two-step incubation immunoassay kit. Recombinant nucleocapsid protein (NP) of SARS-CoV-2 pre-coated onto the polystyrene microwell strips can specifically recognize anti-NP antibodies in human serum or plasma specimens. After a 1 hour incubation, anti-NP antibodies are captured by immobilized NP protein while the unbound components are washed away. Afterwards, a detection solution containing HRP-conjugated anti-human antibody is added for another 1 hour incubation, wherein HRP-conjugated anti-human antibody binds to the antibodies previously bound to NP protein on the plate. After removal of nonspecific bindings, an HRP substrate solution containing 3,3',5,5'-Tetramethylbenzidine (TMB) is added, resulting in the formation of a blue color. Color reaction is stopped by 2M H2SO4, transforming the blue color to yellow, which is quantified by an absorbance microplate reader at 450 nm. Microplate wells with increased color intensity indicate presence of higher amount of anti-NP antibodies in the tested samples.
This product is intended for use by professional persons only. For research use only. Not for use in clinical & diagnostic procedures. In line with FDA guidelines, test reports must include the following statements:
(1) This test has not been reviewed by the FDA.
(2) Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus.
(3) Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
(4) Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
(5) Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. -
Related Products
SARS-CoV-2 Nucleocapsid protein (NP), Human IgM, ELISA assay
SARS-CoV-2 Nucleocapsid Protein (NP), Purified Recombinant Protein
SARS-CoV-2 Spike Protein S1 Receptor-Binding Domain (S1RBD), Purified Recombinant Protein
- Application(s) ELISA
-
Application Details
ELISA. For the detection of SARS-CoV-2 Nucleocapsid protein, Total Human IgG (NP) in Serum, Plasma (Citrate), Plasma (EDTA), Plasma (Heparin). Please download the detailed product insert for complete instructions for the successful use of this ELISA. Use only as directed.
This product is intended for use by professional persons only. For research use only. Not for use in clinical & diagnostic procedures. In line with FDA guidelines, test reports must include the following statements:
(1) This test has not been reviewed by the FDA.
(2) Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus.
(3) Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
(4) Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
(5) Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. - Target SARS-CoV-2 Nucleocapsid protein, Total Human IgG (NP)
- Specificity Specificity: 95.0 % (n = 180)/Sensitivity: 93.3 % (n = 60);Clinical validation study of the Biosensis SARS-CoV-2 NP Total Antibody ELISA was conducted in 2020 in Shenzhen, China. Samples were collected from COVID-19 confirmed cases with clinical symptoms, laboratory abnormalities or pulmonary imaging manifestations. No tests have been performed on specimens from latent infections or patients in the incubation period. The kit showed higher positive detection rate in specimens from patients with delayed onset. Therefore, the interpretation of the test results should consider the specimen s collection time. It is highly recommended that each laboratory establishes its own normal and pathological reference range for anti-NP antibody level. Furthermore, it is also recommended that each laboratory includes its own panel of control samples in the assay.
- Target Host Species Human
- Species Reactivity Human
- Sample Type Plasma (Citrate), Plasma (EDTA), Plasma (Heparin), Serum
- Detection Method Colorimetric
- Kit Components The ELISA kit box contains 96-well pre-coated strip plate(s), blank control sample, detection reagents, wash and sample buffers, substrate buffer and detailed protocols.
- Storage Instructions 2-8°C. Product ships on ice packs and is stable from ambient to refrigerated temperatures for 1 week.
- Storage Temperature 2-8°C
- Batch Number Please see item label.
- Expiration Date The unopened kit is stable for 6 months from date of receipt when stored at 2-8°C. Once opened, store reagents at 2-8°C for up to 1 month.
- Alternative Names Nucleocapsid phosphoprotein [Severe acute respiratory syndrome coronavirus 2]; SARS-CoV-2;
- Uniprot Number P0DTC9
- Uniprot Number/Name P0DTC9 (NCAP_SARS2)
- Shipping Statement For order quantities larger than 1 plate, this product is supplied in package units of 2 plates.
- Shipping Temperature 2-8°C (on cold packs)
- UNSPSC CODE 41116158
- Regulatory Status For research use only.

 1800 605-5127
1800 605-5127 +61 (0)8 8352 7711
+61 (0)8 8352 7711