Amyloid beta peptide (A-beta 40/42), Mouse Monoclonal Antibody (Biotin)
- Product Name Amyloid beta peptide (A-beta 40/42), Mouse Monoclonal Antibody (Biotin)
-
Product Description
Mouse anti-Amyloid beta peptide (A-beta 40/42) Monoclonal Antibody (Biotin), suitable for WB, IHC-Frozen, IHC-Paraffin-embedded, ICC, IP, ELISA.
- Alternative Names Beta-APP42; Beta-APP40; Beta-amyloid protein 42; Beta-amyloid protein 40; ABPP; APPI; Amyloid beta A4 protein; MOAB2; MOAB-2; Alzheimer's antibody; AB40; AB42; abeta
- Application(s) ELISA, ICC, IHC-Frozen, IHC-Paraffin-embedded, IP, WB
- Antibody Host Mouse
- Antibody Type Monoclonal
- Specificity MOAB-2 detects preparations enriched in U-, O-, F-Aβ42, and U-Aβ40 by dot-blot, and is thus a pan-specific Aβ antibody. However, MOAB-2 is selective for the more neurotoxic Aβ42 compared to Aβ40. Indeed, MOAB-2 demonstrated a titration against antigen concentration, and detects Aβ40 at 2.5 pmol, but U-, O- and F-Aβb42 at antigen concentrations as low as ~ 0.1 pmol (Youmans. KL et al., 2012; PMID: 22423893). MOAB-2 does not detect APP (Amyloid Precursor Protein). Human, rat, other species not yet tested. By Dot Blot, MOAB-2 detected rat Aβ40 and human Aβ40, albeit with less affinity than for Aβ42 (Youmans KL et al., 2012).
- Species Reactivity Human, Rat
- Immunogen Description Recombinant human amyloid beta protein 42 (Aβ42): DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA
- Conjugate Biotin
- Purity Description Antibody was purified from cell culture supernatant by Protein G chromatography, biotinylated and buffer-exchanged into PBS, pH 7.4 buffer
- Regulatory Status For research use only.
Product Info
-
Product Description
Mouse anti-Amyloid beta peptide (A-beta 40/42) Monoclonal Antibody (Biotin), suitable for WB, IHC-Frozen, IHC-Paraffin-embedded, ICC, IP, ELISA.
-
Related Products
Oligomeric Amyloid-beta, Human, Rat, ELISA assay
Amyloid beta peptide (A-beta 40/42), Mouse Monoclonal Antibody
- Application(s) ELISA, ICC, IHC-Frozen, IHC-Paraffin-embedded, IP, WB
-
Application Details
The biotinylated MOAB-2 antibody has been tested by IHC (1:500 - 1:2,000 dilution) and is also expected to work in applications validated for the unlabelled antibody (M-1586-100) at same or higher dilutions: Western Blotting (WB), Immunohistochemistry (IHC), Immunohistochemistry/paraffin embedded IHC(P), Immunoprecipitation (IP), Immunofluorescence (IF), ELISA.
Western Blotting:
MOAB-2 has been tested in WB using purified synthetic beta-amyloid preparations and from transgenic mouse brain formic acid extracts (see Figure 1). Formic acid extraction/concentration is required for western blot detection from extracts. Suggested dilution of 1:2000-1:5,000 for WB, standard ECL detection systems.
Tissue samples for the detection of beta-amyloid should be prepared as detailed in Youmans KL et al., 2011 (Journal of Neuroscience Methods 196: 51-59) for best results. Detection of beta-amyloid 40/42 in direct westerns can be difficult; Dot-blots of prepared samples are recommended as detailed in Youmans KL et al., 2012.
Immunohistochemistry:
Suggested dilution for biotinylated MOAB-2 in IHC is 1:500-1:2,000. Fresh frozen, 4% paraformaldehyde fixed frozen, or formalin fixed paraffin embedded tissues are all suitable. Antigen retrieval is required in fixed tissues for optimal staining.
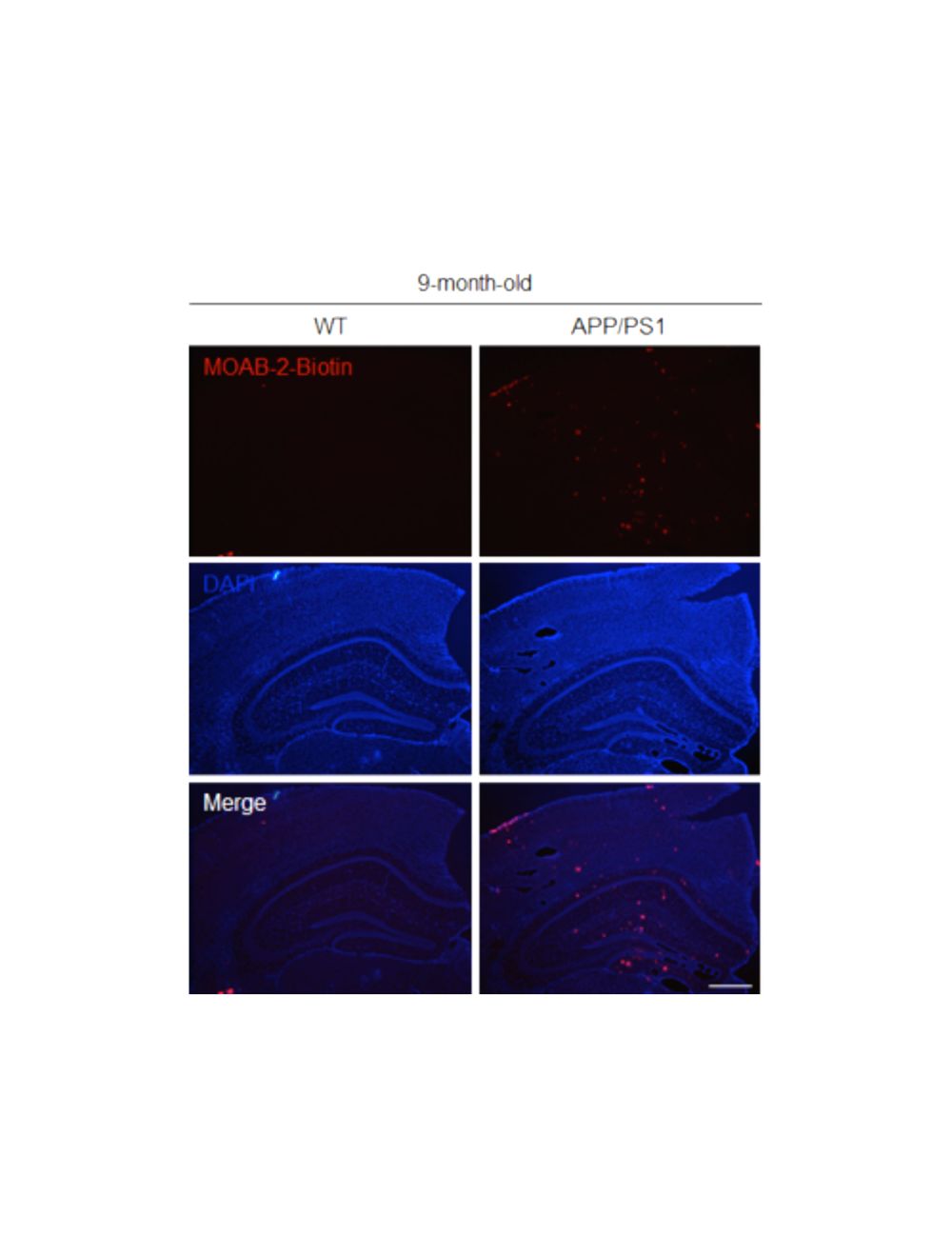
Antibody was tested on 4% paraformaldehyde/0.1% glutaraldehyde fixed frozen tissue from 3xTg and 5xFAD mice. MOAB-2 antibody detects intraneuronal and extracellular beta-amyloid in IHC and does not detect APP (Youmans KL et al., 2012).
The antibody also reacts with archival formalin-fixed, paraffin-embedded tissue samples with antigen Heat Induced Epitope Retrieval (HIER). Recommended buffer for HIER is citrate, pH 6.0. Signal was weak without antigen retrieval. Immunoreactivity was observed in intraneural-amyloid deposition (plaque) in Alzheimer's brain. MOAB-2 was found to be extremely clean and with an excellent signal to noise ratio with no neuro-cellular diffusive staining.
In addition, MOAB-2 demonstrated no significant differences in A-beta detection using paraffin fixed, free-floating sections (Youmans KL et al., 2012). Formic acid (FA) treatment resulted in optimal detection of both intraneuronal and extracellular A-beta compared to without FA (incubated in 88% FA 8 min, Youmans KL et al., 2012). Free floating tissue sections were permeabilized in TBS containing 0.25% Triton X-100 (TBSX; 3 x 10 min), blocked with 3% horse serum in TBSX (3 x 10 min) followed by 1% horse serum in TBSX (2 x10 min) and incubated with appropriate primary antibodies diluted in TBSX containing 1% horse serum overnight. See Youmans KL et al., 2012, for full IHC(P) protocol and method details.
Immunofluorescence:
For IF, suggested dilution is 1:100-1:500. The antibody was tested on 4% PFA fixed frozen tissue. Fixed tissues were washed in TBS (3 x 10 min), then incubated in 88% FA (8 min), and then permeabilized in TBSX (3 x 10 min), and blocked in TBSX containing 5% bovine serum albumin (BSA; 1 hr). Sections were subsequently incubated with appropriate primary antibodies diluted in TBSX containing 2% BSA overnight on an oscillatory rotator. Detection was via fluorescently labelled absorbed secondary antibodies (Youmans KL et al., 2012).
Immunoprecipitation:
For IP, the suggested dilution is 1:200 to 1:1,000 for labelled beta-amyloid using SA-coated beads as the capture vehicle, similar to the protocols employed by Youmans KL et al., 2012.
ELISA:
In an ELISA, a dilution of 1:50-1:1,000 is suggested. The antibody has been tested in ELISAs on synthetic beta-amyloid and tissue homogenates from beta-amyloid-Tg mice.
Biosensis recommends optimal dilutions/concentrations should be determined by the end user for all applications. Dilutions provided are only meant to serve as a basic guide. - Target Amyloid beta peptide (A-beta 40/42)
- Specificity MOAB-2 detects preparations enriched in U-, O-, F-Aβ42, and U-Aβ40 by dot-blot, and is thus a pan-specific Aβ antibody. However, MOAB-2 is selective for the more neurotoxic Aβ42 compared to Aβ40. Indeed, MOAB-2 demonstrated a titration against antigen concentration, and detects Aβ40 at 2.5 pmol, but U-, O- and F-Aβb42 at antigen concentrations as low as ~ 0.1 pmol (Youmans. KL et al., 2012; PMID: 22423893). MOAB-2 does not detect APP (Amyloid Precursor Protein). Human, rat, other species not yet tested. By Dot Blot, MOAB-2 detected rat Aβ40 and human Aβ40, albeit with less affinity than for Aβ42 (Youmans KL et al., 2012).
- Target Host Species Human
- Species Reactivity Human, Rat
- Antibody Host Mouse
- Antibody Type Monoclonal
- Antibody Isotype IgG2b, lambda
- Clone Name MOAB-2
- Conjugate Biotin
- Immunogen Description Recombinant human amyloid beta protein 42 (Aβ42): DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA
- Purity Description Antibody was purified from cell culture supernatant by Protein G chromatography, biotinylated and buffer-exchanged into PBS, pH 7.4 buffer
- Format Lyophilized from PBS buffer, pH 7.4; contains no preservative.
- Reconstitution Instructions Spin vial briefly before opening. Reconstitute in 50 µL sterile-filtered, ultrapure water to give a concentration of 1 mg/mL. Centrifuge to remove any insoluble material. Final buffer is PBS, pH 7.4 without preservative.
- Storage Instructions After reconstitution keep aliquots at -20°C to -70°C for a higher stability. At 2-8°C keep up to one week; use sterile methods and pipettes. Highly purified glycerol (1:1) may be added for an additional stability. Avoid repetitive freeze/thaw cycles. Keep tightly closed when not in use and protected from light.
- Batch Number Please see item label.
- Expiration Date 12 months after date of receipt (unopened vial).
- Alternative Names Beta-APP42; Beta-APP40; Beta-amyloid protein 42; Beta-amyloid protein 40; ABPP; APPI; Amyloid beta A4 protein; MOAB2; MOAB-2; Alzheimer's antibody; AB40; AB42; abeta
- Uniprot Number P05067
- Uniprot Number/Name P05067 (A4_HUMAN)
-
Scientific Background
The amyloid beta peptide is derived from the cleavage of the Amyloid precursor protein (APP) and varies in length from 39 to 43 amino acids. However, the form(s) of amyloid-beta peptide (Aβ associated with the pathology characteristic of Alzheimer's disease (AD) remains unclear. In particular, the neurotoxicity of intraneuronal Aβ accumulation is an area of considerable research and controversy principally because antibodies thought to be specific for Aβ have been shown to actually detect intraneuronal APP and not Aβ exclusively.
MOAB-2 (mouse IgG2b) is a pan-specific, high-titer antibody to Aβ residues 1-4 as demonstrated by biochemical and immunohistochemical analyses (IHC), and is highly specific just to amyloid beta peptide. MOAB-2 did not detect APP or APP-CTFs in cell culture media/lysates (HEK-APPSwe or HEK APPSwe/BACE1) or in brain homogenates from transgenic mice expressing 5 familial AD (FAD) mutation (5xFAD mice).
Using IHC on 5xFAD brain tissue, MOAB-2 immunoreactivity co-localized with C-terminal antibodies specific for Aβ40 and Aβ42. MOAB-2 did not co-localize with either N- or C-terminal antibodies to APP. In addition, no MOAB-2-immunreactivity was observed in the brains of 5xFAD/BACE-/- mice, although significant amounts of APP were detected by N- and C-terminal antibodies to APP, as well as by 6E10. In both 5xFAD and 3xTg mouse brain tissue, MOAB-2 co-localized with cathepsin-D, a marker for acidic organelles, further evidence for intraneuronal Aβ, distinct from Aβ associated with the cell membrane. MOAB-2 demonstrated strong intraneuronal and extra-cellular immunoreactivity in 5xFAD and 3xTg mouse brain tissues.
Biosensis now offers biotinylated MOAB-2 antibody allowing more flexibility in experimental design by using the biotin-avidin/streptavidin detection method. Biotinylated MOAB-2 antibody may also help to reduce background staining in difficult-to-stain tissues and increase detection sensitivity. The ability of biotinylated MOAB-2 antibody to detect amyloid beta has been validated by IHC.
Purified, non-biotinylated MOAB-2 antibody is available here. - Shipping Temperature 25°C (ambient)
- UNSPSC CODE 41116161
- Regulatory Status For research use only.
Specifications
-
Specific References
Tang X. et al. (2023) ATG5 (autophagy related 5) in microglia controls hippocampal neurogenesis in Alzheimer disease Autophagy. [Epub ahead of print] Application: IHC
Kim, S. et al. (2020) Performance Validation of a Planar Hall Resistance Biosensor through Beta-Amyloid Biomarker. Sensors (Basel). 20(2) Application: In-vitro biosensor.
Ruan, CS. et al. (2017) Sortilin inhibits amyloid pathology by regulating non-specific degradation of APP. Exp Neurol. [Epub ahead of print] Application: IHC
References for non-biotinylated MOAB-2 antibody (M-1586-100):
Zhu, B. et al. (2017) ER-associated degradation regulates Alzheimer's amyloid pathology and memory function by modulating _-secretase activity. Nat Commun. 8(1):1472. Application: IHC
Huang, TY. et al. (2017) SORLA attenuates EphA4 signaling and amyloid _-induced neurodegeneration. J Exp Med. pii: jem.20171413. [Epub ahead of print]. Application: IHC
Felecia, M. et al. (2017) Peripheral Inflammation, Apolipoprotein E4, and Amyloid-_ Interact to Induce Cognitive and Cerebrovascular Dysfunction. ASN Neuro. 9(4):1759091417719201. Application: IHC/IF
Thomas, R. et al. (2016) Epidermal growth factor prevents APOE4 and amyloid-beta-induced cognitive and cerebrovascular deficits in female mice. Acta Neuropathol Commun. 4(1):111 Application: IHC
Koster, KP. et al. (2016) Epidermal growth factor prevents oligomeric amyloid-_ induced angiogenesis deficits in vitro. J Cereb Blood Flow Metab. [Epub ahead of print] Application: IF
Loffler, T. et al. (2016) Decreased Plasma Aβ in Hyperlipidemic APPSL Transgenic Mice Is Associated with BBB Dysfunction. Front. Neurosci. Application: IF
Kobro-Flatmoen, A. et al. (2016) Reelin-immunoreactive neurons in entorhinal cortex layer II selectively express intracellular amyloid in early Alzheimer's disease. Neurobiology of Disease. 93:172-183. Application: IHC
Tai, LM. et al. (2016) The role of APOE in cerebrovascular dysfunction. Acta Neuropathol. 131(5):709-23. Application: IF
Kim, YH. et al. (2015) A 3D human neural cell culture system for modeling Alzheimer's disease. Nat Prot. 10(7):985-1006. Application: WB
Condello, C. et al. (2015) Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat Commun. 6:6176. Application: IF
Iulita MF et al (2014) Intracellular Abeta pathology and early cognitive impairments in a transgenic rat model overexpressing human amyloid precursor protein: a multidimensional study. Acta Neuropathol Commun. 6:61. Application: IF, IH
Smith BR et al (2014) Neuronal inclusions of alpha-synuclein contribute to the pathogenesis of Krabbe disease. J Pathol. Apr;235(5):509-21. Application: IF -
General References
Tai LM et al (2016) "The role of APOE in cerebrovascular dysfunction."Acta Neuropathol. 2016 Feb 16. [Epub ahead of print]
Tai LM et al (2013) Levels of soluble apolipoprotein E/amyloid-_ (Aβ) complex are reduced and oligomeric Aβ increased with APOE4 and Alzheimer disease in a transgenic mouse model and human samples. J Biol Chem. 2013 Feb 22;288(8):5914-26.
K.L. Youmans et al (2012) Intraneuronal Abeta detection in 5xFAD mice by a new Abeta-specific antibody Mol Neurodegener. 2012 Mar 16;7(1):8.
K.L. Youmans et al (2011) Amyloid-_42 alters apolipoprotein E solubility in brains of mice with five familial AD mutations J Neurosci Methods. 2011 Mar 15;196(1):51-9.

 1800 605-5127
1800 605-5127 +61 (0)8 8352 7711
+61 (0)8 8352 7711